Primer 2: Systems-Based Approaches to Diagnostic Excellence
Summary
This is Part 2 of a three-part series of primers dedicated to diagnostic error. Part 1 focuses on foundational concepts of diagnostic error. This primer focuses on systems-based approaches to diagnostic excellence. Part 3 focuses on the role of clinical reasoning in diagnostic excellence
Background
Diagnostic errors – missed, delayed, or incorrect diagnoses – are estimated to affect every person at some point during their lifetime.1 At the systems-level, accurate identification of interventions to decrease diagnostic error remain top priorities for organizations pursuing diagnostic excellence.2 This primer reviews current available strategies to detect and monitor diagnostic errors and discusses systems-based interventions to improve diagnostic safety including organizational initiatives and optimization of health information technology (HIT). Together, these systems-based interventions aim to reduce diagnostic errors by improving decision-making processes and fostering more accurate and timely diagnoses.
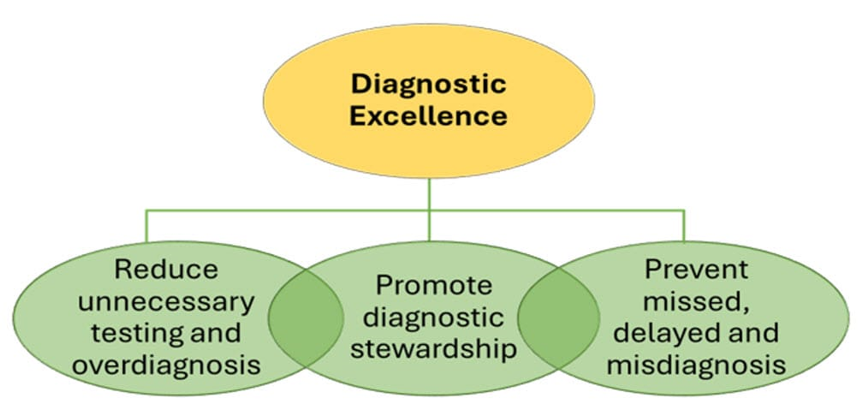
Figure 1 shows the pathway to improving diagnostic excellence by providing a framework to target improvement efforts. The complexity involved in identifying and tracking diagnostic errors underscores the need for thoughtful diagnostic stewardship. This means ordering the right evaluations for the right patients at the right time to ensure a balanced use of diagnostic modalities in addition to preventing missed and delayed diagnoses.
Figure 1. Core Elements of Diagnostic Excellence Programs
Source: Reproduced from the Centers for Disease Control and Prevention (CDC). 2
Measurement
Accurate measurement of diagnostic performance is necessary for systems-level efforts to mitigate diagnostic error. However, tracking diagnostic safety events is complex and challenging involving systems-level factors, such as inappropriate test ordering, missed or misinterpreted symptoms, and communication breakdowns. It also requires assessing consequences ranging from unnecessary treatments to delayed diagnosis and potential patient harm.
There are established frameworks for the detection of adverse diagnostic safety events which can be employed to identify diagnostic errors, monitor for errors, and measure the incidence of specific types of errors by condition or disease process.
- SPADE (Symptom-Analysis Pair Analysis of Diagnostic Error) [identification, measurement of certain disease processes]: Retrospective statistical analysis methodology to analyze large patient databases (without human adjudication) by linking specific pairs of symptoms at high risk of leading to misdiagnoses (eg, dizziness) to diagnoses made at a later date (eg, dizziness à la acute stroke at subsequent encounter) to infer diagnostic error-related harm. Employed in research for certain disease states, it may understate the frequency of diagnostic error and is labor intensive. It can, however, provide useful organization or institution-level surveillance for certain disease-specific events.3
- SaferDx [identification]: Organizational framework for diagnostic safety which includes a validated chart review instrument to perform manual evaluations of patient charts for the presence of a diagnostic error. This method can detect diagnostic error but is labor intensive – making it difficult to employ for large-scale error measurement.4
- Review of autopsy databases for diagnostic errors [identification]: Changes in diagnosis based on post-mortem pathology results provide information on how often diagnostic error may contribute to patient death.5
- Review of malpractice claim databases [identification] for diagnostic error-based malpractice claims provide insight into the financial burden associated with diagnostic errors.6,7 Validated survey instruments for both clinicians and patients can provide detailed accounts of specific diagnostic safety events [monitoring]. Clinician surveys often focus on process breakdowns, while patient surveys (eg, AHRQ – Toolkit for Engaging Patients to Improve Diagnostic Safety8) capture the patient experience, including missed opportunities that may not be otherwise known. This patient-centered approach promotes unique perspectives on diagnostic safety events that may not be captured through current patient safety event reporting systems.9
- Individual and institutional self-reporting tools [identification, monitoring, measurement] can provide nuanced information on specific diagnostic safety events. When employed at the organizational level or across healthcare systems such as AHRQ’s Common Formats10 for Event Reporting – Diagnostic Safety Version 1.0E, data on diagnostic safety-related information can be collected, aggregated, and analyzed at the national level.
- Organizational toolkits [identification, monitoring, measurement] such as Measure Dx11 can aid institutions in the set-up and implementation of systems to track and learn from diagnostic safety events at the systems-level.
Interventions
There is a plethora of potential interventions to improve diagnostic safety, but it should be noted that undifferentiated interventions may not be effective in decreasing diagnostic error.12 This underscores the need for targeted, proven interventions at the bedside and system levels. The National Academies of Sciences, Engineering, and Medicine (NASEM) Improving Diagnosis in Health Care1 report in 2015 provide eight recommendations to improve diagnosis in healthcare. These are listed in Table 1 along with added links to recommendation-specific resources. From a systems perspective, there are various types of interventions that organizations can implement to improve diagnostic safety.
Table 1. Eight Recommendations from the 2015 NASEM Report on Improving Diagnosis in Health Care with the Addition of Links to Additional Resources1
|
|
Recommendation |
Example(s) of application |
|
1 |
Facilitate more effective teamwork in the diagnostic process among health care professionals, patients, and their families. |
|
|
2 |
Enhance healthcare professional education and training in the diagnostic process. |
|
|
3 |
Ensure that health information technologies support patients and health care professionals in the diagnostic process. |
|
|
4 |
Develop and deploy approaches to identify, learn from, and reduce diagnostic errors and near misses. |
|
|
5 |
Establish a work system and culture that supports the diagnostic process and improvements in diagnostic performance. |
|
|
6 |
Develop a reporting environment and medical liability system that facilitates improved diagnosis by learning from diagnostic errors. |
|
|
7 |
Design a payment and care delivery environment that supports the diagnostic process. |
|
|
8 |
Provide dedicated funding for research on the diagnostic process and diagnostic errors. |
Health Information Technology
Health information technology (HIT) plays a crucial role in reducing diagnostic errors by supporting communication across healthcare teams. However, implementing HIT systems requires careful considerations of both system benefits and potential risks. While basic functionalities like billing or documentation are important, HIT must be thoughtfully designed to support diagnostic decision-making without creating new sources of error.
The integration of Diagnostic Decision Support Systems (DDSS) within electronic health records (EHRs) can provide clinicians with real-time, evidence-based recommendations to manage cognitive load, especially when diagnosing complex conditions or rare diseases.19 DDSSs use large databases of clinician information to create differential diagnoses based on patient data. However, these systems must be implemented with careful attention to workflow integration, alert design, and validation of recommendations to avoid over-reliance on the automation.28 Similarly, while AI-enabled tools show promise in diagnostic support through image analysis, pattern recognition, and predictive modeling, their limitations and potential biases must be thoroughly evaluated before implementation in clinical settings.
Clinical decision support tools can help reduce alert fatigue. While traditional alert systems can overwhelm clinicians with excessive notifications, well-designed tools can prioritize high-risk situations by making alerts more actionable.14 The overall goal is to deliver accurate and timely information while preventing alert fatigue and maintaining clinician autonomy in decision-making.
For successful integration, new technologies (eg, HIT, DDSS, AI) require human-centered design approaches that consider both benefits and risks. Involving end users throughout the design, evaluation, and implementation process helps ensures that technology will support, rather than hinder, the diagnostic process.29 When thoughtfully designed and implemented, HIT can enhance diagnostic accuracy, reduce errors, and improve patient safety while mitigating risks, such as alert fatigue, automation bias, and over-reliance on AI-driven recommendations.
Organizations
Improving diagnostic safety requires organizational commitment to timely, accurate diagnoses and a mission of error prevention. This commitment must also include efforts to address access and opportunity for optimal health outcomes.1 Leadership plays a crucial role in establishing systems and processes that reduce diagnostic errors, including structured communication, situation monitoring, and collaborative decision-making. A just culture where staff feel safe reporting errors without fear of punishment, is essential for improving diagnostic safety.
- TeamSTEPPS (Team Strategies and Tools to Enhance Performance and Patient Safety30) is both a framework and a program that can enhance diagnostic safety efforts by providing a structure built around four core competencies: leadership, communication, situation monitoring, and mutual support. The TeamSTEPPS for Diagnosis Improvement Course31 specifically applies this framework to address diagnostic errors, offering customizable training modules and assessment tools.
- Diagnostic Management Teams (DMTs) have demonstrated significant improvements in clinical outcomes, particularly in managing complex conditions like coagulation disorders.32 Brashear et al found that cases reviewed by a coagulation DMT were six times more likely to have an established, scientifically based diagnosis compared to those without DMT involvement. Moreover, patients whose cases were reviewed by a coagulation DMT were twice as likely to have a coagulopathy identified versus having no diagnosis.15 Systematic cross-checking among physicians has been shown to reduce adverse events in emergency departments13, further emphasizing the importance of collaborative approaches in enhancing diagnostic safety. By ensuring no diagnostic decision is made in isolation, DMTs reduce the chances of misdiagnosis.14
- "Closing the loop"33 on test results is essential for diagnostic safety. Health systems can implement systems designed to identify patients with abnormal test results or concerning systems that have not received the necessary follow-up and alert physicians when follow-up actions are missed. This is especially critical for conditions that may progress slowly, where the impact of a delayed diagnosis can be significant. These systems have an important role in reducing the risk of diagnostic errors to support timely treatments.
While TeamSTEPPS provides a broad framework for enhancing communication and teamwork across healthcare and DMTs offer a focused, expert-driven approach to improving diagnostic accuracy in particular specialties, an organizational strategy remains crucial. By implementing these approaches along with systems to support closed loop communication regarding test results, healthcare organizations can create a comprehensive, team-based strategy for diagnostic safety. However, this primer does not provide an exhaustive review of organization-based approaches to diagnostic excellence. Additional resources include the recent Leapfrog Group report outlining 22 recommendations to improve diagnostic safety and quality in hospitals and offer more comprehensive organizational guidance.34
Implementation of diagnostic safety efforts should be guided by an organizational level framework. A recent example of a resource for systems-level implementation is the Safer Dx Checklist35 which contains ten expert-reviewed, consensus driven safety practices to address diagnostic error, including recommendations on organizational and leadership accountability for improving diagnosis, involving patients in diagnostic safety work, and developing and implementing an organizational infrastructure for measurement and improvement activities.
Another priority is the development and implementation of an organization-level diagnostic excellence program which can coordinate actionable efforts to improve diagnostic reasoning, testing, and communication. A recent report Core Elements of Hospital Diagnostic Excellence2 by the Centers for Disease Control and Prevention (CDC) in partnership with the Agency for Healthcare Research and Quality (AHRQ) and the Centers for Medicare & Medicaid Services (CMS) provides a programmatic framework for hospitals and healthcare systems to improve diagnostic stewardship and safety. This toolkit includes an assessment instrument for healthcare systems to evaluate the current status of their diagnostic safety work and highlight areas for improvement at the system, clinician, and patient levels. Two national organizations dedicated to improving diagnostic quality and safety are the UCSF CODEX (Coordinating Center for Diagnostic Excellence) and the new non-profit Community Improving Diagnosis in Medicine (CIDM). UCSF CODEX hosts an annual national conference with CIDM and institutional partners focusing on improving diagnosis.
Conclusion
While there are many promising systems-based interventions to improve diagnosis, most are relatively early in development and lack conclusive evidence of their effectiveness. As the patient safety movement continues its work to improve diagnostic safety, continued efforts and additional research are needed to improve the methodology to measure the frequency of and harm caused by diagnostic errors as well as implement and evaluate systems-based interventions to improve diagnosis in healthcare.
___
Authors
Grant Shafer, MD, MA, FAAP
Neonatologist
CHOC Children’s Hospital
PIH Health Whittier Hospital
[email protected]
Bat-Zion Hose, PhD
Human Factors and Systems Engineer
National Center for Human Factors in Healthcare
MedStar Health Research Institutes
Assistant Professor
Georgetown University School of Medicine
[email protected]
Roslyn Seitz, MSN, MPH
Associate Editor, AHRQ’s Patient Safety Network (PSNet)
Health Sciences Assistant Clinical Professor
University of California, Davis
[email protected]
Mark L Graber, MD, FACP
Founder, Community Improving Diagnosis in Medicine (CIDM)
Professor Emeritus, Stony Brook University, NY
[email protected]
This primer was funded under contract number 75Q80119C00004 from the Agency for Healthcare Research and Quality (AHRQ), U.S. Department of Health and Human Services. The authors are solely responsible for this report’s contents, findings, and conclusions, which do not necessarily represent the views of AHRQ. Readers should not interpret any statement in this report as an official position of AHRQ or of the U.S. Department of Health and Human Services. None of the authors has any affiliation or financial involvement that conflicts with the material presented in this report.
Publication date: 6/25/2025
___
References
1. Balogh EP, Miller BT, Ball JR, eds. Committee on Diagnostic Error in Health Care, Board on Health Care Services, Institute of Medicine, The National Academies of Sciences, Engineering, and Medicine. Improving Diagnosis in Health Care. National Academies Press; 2015.
2. Core Elements of Hospital Diagnostic Excellence (DxEx). Patient Safety. US Centers for Disease Control and Prevention. September 17, 2024. Accessed May 28, 2025. https://www.cdc.gov/patient-safety/hcp/hospital-dx-excellence/index.html
3. Liberman AL, Newman-Toker DE. Symptom-Disease Pair Analysis of Diagnostic Error (SPADE): a conceptual framework and methodological approach for unearthing misdiagnosis-related harms using big data. BMJ Qual Saf. 2018;27(7):557-566.
4. Singh H, Bradford A, Goeschel C. Operational measurement of diagnostic safety: state of the science. Diagnosis (Berl). 2021;8(1):51-65.
5. Shojania KG, Burton EC, McDonald KM, Goldman L. Changes in rates of autopsy-detected diagnostic errors over time: a systematic review. JAMA. 2003;289(21):2849-2856.
6. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-Year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680.
7. Newman-Toker DE, Schaffer AC, Yu-Moe CW, et al. Serious misdiagnosis-related harms in malpractice claims: The “Big Three” - vascular events, infections, and cancers. Diagnosis (Berl). 2019;6(3):227-240.
8. Agency for Healthcare Research and Quality. Toolkit for Engaging Patients To Improve Diagnostic Safety. Accessed May 28, 2025. https://www.ahrq.gov/diagnostic-safety/tools/engaging-patients-improve…
9. Kaur AP, Levinson AT, Monteiro JFG, Carino GP. The impact of errors on healthcare professionals in the critical care setting. J Crit Care. 2019;52:16-21.
10. Agency for Healthcare Research and Policy. PSO Privacy Protection Center: Diagnostic Safety 1.0. Accessed May 28, 2025. https://www.psoppc.org/psoppc_web/publicpages/commonFormatsDSV1.0
11. Measure Dx: A Resource To Identify, Analyze, and Learn From Diagnostic Safety Events. Accessed June 16, 2025. https://www.ahrq.gov/diagnostic-safety/tools/measure-dx.html
12. Ahsani-Estahbanati E, Sergeevich Gordeev V, Doshmangir L. Interventions to reduce the incidence of medical error and its financial burden in health care systems: A systematic review of systematic reviews. Front Med (Lausanne). 2022;9:875426.
13. Freund Y, Goulet H, Leblanc J, et al. Effect of systematic physician cross-checking on reducing adverse events in the emergency department: the CHARMED cluster randomized trial. JAMA Intern Med. 2018;178(6):812-819.
14. Singh H, Mushtaq U, Marinez A, et al. Developing the Safer Dx Checklist of ten safety recommendations for health care organizations to address diagnostic errors. Jt Comm J Qual Patient Saf. 2022;48(11):581-590.
15. Brashear J, Mize R, Laposata M, Zahner C. Impact of diagnostic management team on patient time to diagnosis and percent of accurate and clinically actionable diagnoses. Diagnosis (Berl). 2024;11(2):132-135.
16. Meyer FML, Filipovic MG, Balestra GM, Tisljar K, Sellmann T, Marsch S. Diagnostic errors induced by a wrong a priori diagnosis: a prospective randomized simulator-based Trial. J Clin Med. 2021;10(4):826.
17. Kassirer JP. Our stubborn quest for diagnostic certainty. a cause of excessive testing. N Engl J Med. 1989;320(22):1489-1491.
18. Meyer AND, Giardina TD, Khawaja L, et al. Patient and clinician experiences of uncertainty in the diagnostic process: current understanding and future directions. Patient Educ Couns. 2021;104(11):2606-2615.
19. Miller K, Biro J, Gold JA, et al. Documenting Diagnosis: Exploring the Impact of Electronic Health Records on Diagnostic Safety. Rockville, MD: Agency for Healthcare Research and Quality; August 2024 AHRQ Publication No 24-0010-3-EF.
20. Murphy DR, Wu L, Thomas EJ, et al. Electronic trigger-based intervention to reduce delays in diagnostic evaluation for cancer: a cluster randomized controlled trial. J Clin Oncol. 2015;33(31):3560-3567.
21. Dave N, Bui S, Morgan C, et al. Interventions targeted at reducing diagnostic error: systematic review. BMJ Qual Saf. 2022;31(4):297-307.
22. Manners J, Khandker N, Barron A, et al. An interdisciplinary approach to inhospital stroke improves stroke detection and treatment time. J Neurointerv Surg. 2019;11(11):1080-1084.
23. Ratwani RM, Bates DW, Gold J. Addressing electronic health record contributions to diagnostic error. Health Affairs Forefront. April 25, 2024. Accessed May 28, 2025.
24. Agency for Healthcare Research and Quality. Diagnostic Safety Centers of Excellence. Accessed May 28, 2025. https://www.ahrq.gov/patient-safety/diagnostic-excellence-grants/index…
25. National Academy of Medicine. Fellowship Program - NAM. October 23, 2024. Accessed May 28, 2025. https://nam.edu/our-work/health-policy-fellowships-and-leadership-progr…
26. American Board of Medical Specialties I ABMS. September 15, 2020. Accessed May 28, 2025. https://www.abms.org/
27. Patient-Centered Outcomes Research Institute | PCORI. Accessed May 28, 2025. https://www.pcori.org/
28. Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25(1):44-56.
29. Carayon P, Hose BZ, Wooldridge A, et al. Human-centered design of team health IT for pediatric trauma care transitions. Int J Med Inform. 2022;162:104727.
30. Agency for Healthcare Research and Quality. TeamSTEPPS (Team Strategies & Tools to Enhance Performance & Patient Safety). Accessed May 28, 2025. https://www.ahrq.gov/teamstepps-program/index.html
31. Agency for Healthcare Research and Quality. TeamSTEPPS for Diagnosis Improvement. Accessed May 28, 2025. https://www.ahrq.gov/teamstepps-program/diagnosis-improvement/index.html
32. Graber ML, Rusz D, Jones ML, et al. The new diagnostic team. Diagnosis (Berl). 2017;4(4):225-238.
33. Partnership for Health IT Patient Safety. Closing the Loop: Using Health IT to Mitigate Delayed, Missed, and Incorrect Diagnoses Related to Diagnostic Testing and Medication Changes Using Health IT. ECRI; 2018. Accessed May 28, 2025. https://www.ecri.org/Resources/HIT/Closing_Loop/Closing_the_Loop_Toolki…
34. Recognizing Excellence in Diagnosis Report July 2024. Leapfrog Group; 2024. Accessed May 28, 2025. https://www.leapfroggroup.org/sites/default/files/Files/Recognizing%20E…
35. Safer Dx Checklist: 10 High-Priority Practices for Diagnostic Excellence. Baylor College of Medicine; 2022. Accessed May 28, 2025. https://www.ihi.org/resources/tools/safer-dx-checklist-10-high-priority…